Data-driven process modeling and risk assessment in aseptic manufacturing.
We help to ensure the highest standards with data-driven quality risk management built on deep industry expertise.
It’s all about seamless human integration into data-driven processes.
Our Quality Risk Management solution automates process modeling, risk assessment, and documentation, minimizing production risks and time-to-market while ensuring quality and compliance.
The Innerspace Frame-by-Frame® method builds the industry’s most comprehensive process knowledge database—leveraging deep insights into your training, workflows, and process structures in aseptic manufacturing.
62%
62% of drug shortages result from quality issues, and advanced manufacturing can help to address supply disruptions.
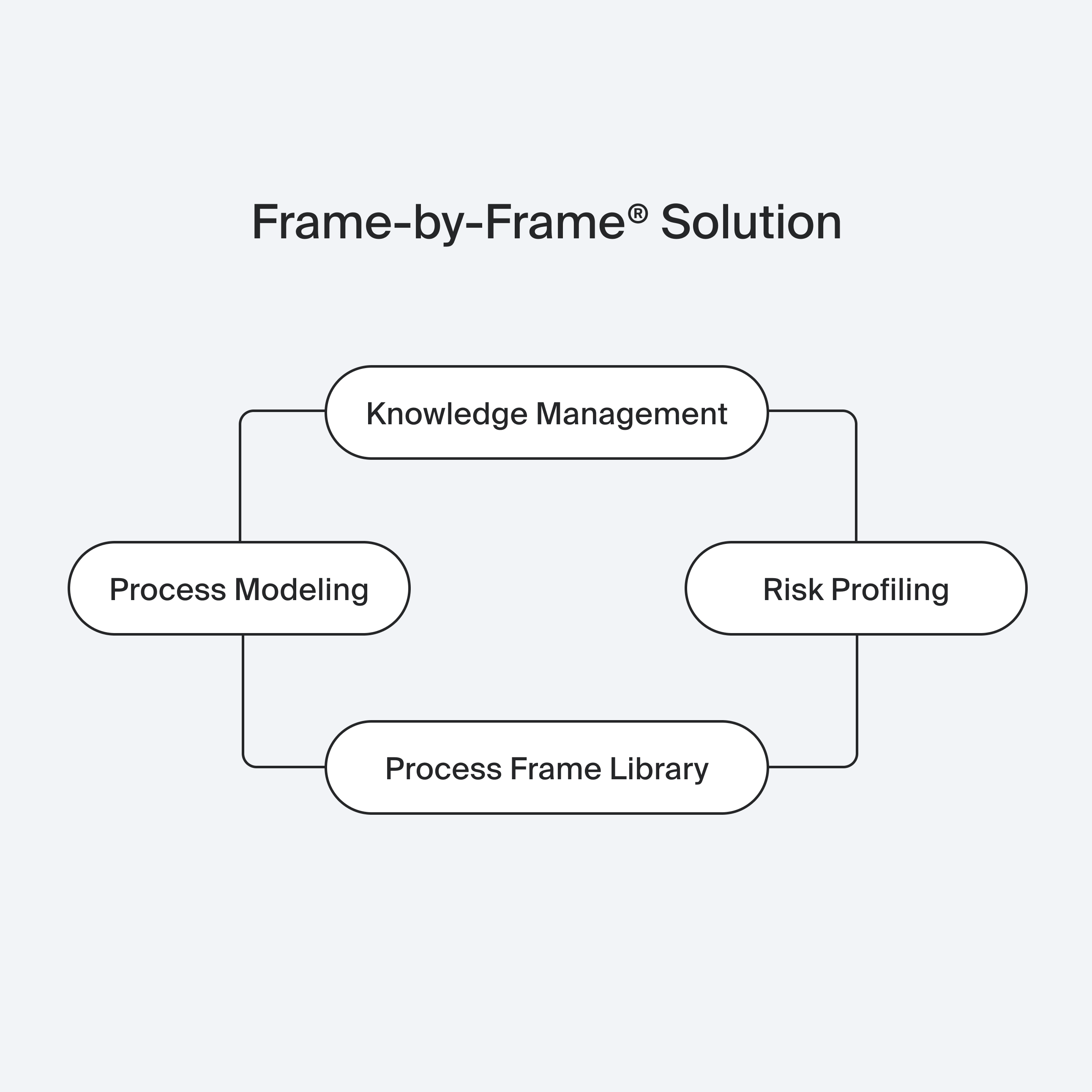
Process Modeling
Data-driven process modeling with Frame-by-Frame® enables precise development and optimization of aseptic manufacturing processes, ensuring higher quality and safety standards.
Risk Assessment
Data-driven risk assessment with Frame-by-Frame® enhances accuracy, consistency, and reliability in aseptic manufacturing. It minimizes subjectivity and ensures a standardized evaluation of risks.
Knowledge Management
Automated document generation with Frame-by-Frame® provides reliable, data-driven compliance documentation, ensuring objectivity, transparency, and consistency across processes and risk assessments.
Safer & Faster Manufacturing
We tackle key challenges in aseptic manufacturing with an automated, data-driven approach to process design, risk assessment, and knowledge management.
Powered by our Process Frame Library, Frame-by-Frame® analyzes thousands of process steps to generate optimized models with minimal risk. Through Risk Profiling and variant comparison, we identify the safest, most efficient process.
Finally, our Knowledge Management system supports implementation with process documentation, quality monitoring, and tailored training.
Interested in automating quality risk management? Contact us to learn how we can assist you.
What the industry says about us
FAQ’s
Frame-by-Frame® is a data-driven technology that systematically analyzes each stage of a pharmaceutical process to identify the highest process risks and potential pitfalls. By delivering objective insights, it enables the implementation of effective mitigation strategies to ensure product quality, safety, and regulatory compliance.
By systematically deconstructing each pharmaceutical process into discrete steps, the so-called process frame, this approach allows for a granular, real-time evaluation of risks at every step. Unlike traditional risk assessment methods, which often rely on retrospective or batch-level evaluations, this approach proactively identifies the highest process risks and provides objective insights for targeted interventions.
Frame-by-Frame® is designed to be adaptable across various pharmaceutical processes, including aseptic and non-aseptic manufacturing, fill-finish operations, packaging, and other quality control workflows.
Frame-by-Frame®, built on ICH Q9 principles, enhances product quality and patient safety by strengthening existing risk management systems. Rather than just integrating, it provides comparative analysis to reinforce previous risk assessments, ensuring more objective and data-driven decision-making.
Frame-by-Frame® collects all relevant data that impacts quality data, including operator behavior, equipment performance, environmental conditions, and process parameters. It systematically links these factors to operational and functional safety, ensuring a comprehensive risk profile that enables real-time identification and mitigation of potential quality risks.
Providing real-time risk detection instead of retrospective analysis.
By identifying common operator errors, process deviations, and best practices, Frame-by-Frame® provides data-driven insights for targeted training programs. It enables pharmaceutical companies to develop more precise SOPs, conduct risk-based training, and continuously improve workforce competency, ultimately reducing human error and strengthening compliance.
SOPs often evolve based on historical deviations, CAPAs, and regulatory updates. On the contrary, Frame-by-Frame® ensures SOP completeness and accuracy by mapping every process step, linking risk factors to specific procedures, and identifying gaps or inconsistencies. This allows companies to continuously refine their SOPs based on real-world data rather than assumptions, improving operational reliability.
Frame-by-Frame® generates a comprehensive, real-time risk profile that supports audit readiness by providing:
This ensures pharmaceutical manufacturers can confidently present their risk management framework during inspections, minimizing compliance risks.
We’d love to hear from you
Fill out the form, and one of our experts will get back to you shortly. Whether you have a question or need assistance, we’re here to help.


